21+ Pdufa Vii Commitment Letter

Prescription Drug User Fee Act Pdufa Vii And Type D Meetings
Growing Complex Generic Application Load May Force Us Fda Staffing Changes Pink Sheet

Pdufa Vii A Vital Reauthorization Youtube

Us Fda S Real Time Oncology Review Program Is No Guarantee For Early Approval Pink Sheet
Pregnancy Safety Could Transform Us Fda Sentinel Role In Post Marketing Studies Pink Sheet
Pdufa Vii Will Fund Pregnancy Postmarket Safety Update Sentinel Upgrades Rems Standardization Pink Sheet

Ddv Letter Of Commitment Relating To The Processing Of Personal Data

Regulatory Guidance Monthly Review Aug 2021

Fda User Fee Programs Reauthorized Fda S Cber Is A Clear Winner

Fda Advances Patient Engagement Via Pdufa Vii Performance Goals Publications Insights Faegre Drinker Biddle Reath Llp
Fda User Fee Reauthorization Ensuring Safe And Effective Drugs And Biologics 02 03 2022 Fda
Inline Xbrl Viewer

Pdufa Vii Is Critical For Future Biopharmaceutical Innovation And For Patients

Fda Announces Cmc Review Pilot For Drugs With Expedited Development Raps

High Quality Communication Enables High Quality Drug Products

Pdufa Vii A Vital Reauthorization Research America

Rare Disease Community Wins In Pdufa Vii Everylife Foundation For Rare Diseases
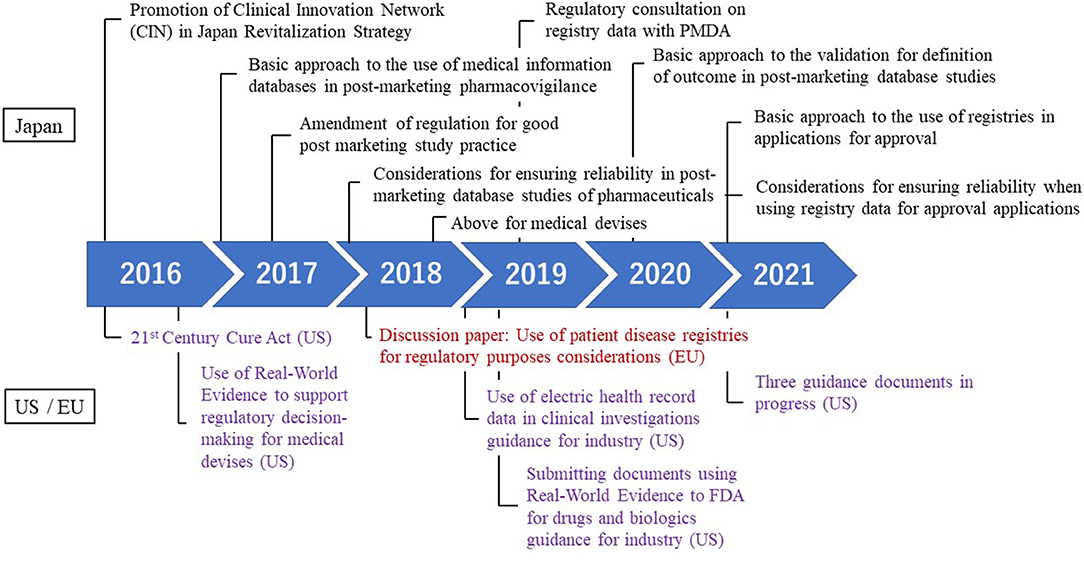
Frontiers Regulatory Approval With Real World Data From Regulatory Science Perspective In Japan